Now, mask factories in European and American countries are facing some pressure: on the one…
Guidance for the Purchased Masks to Pass the China Customs Successfully
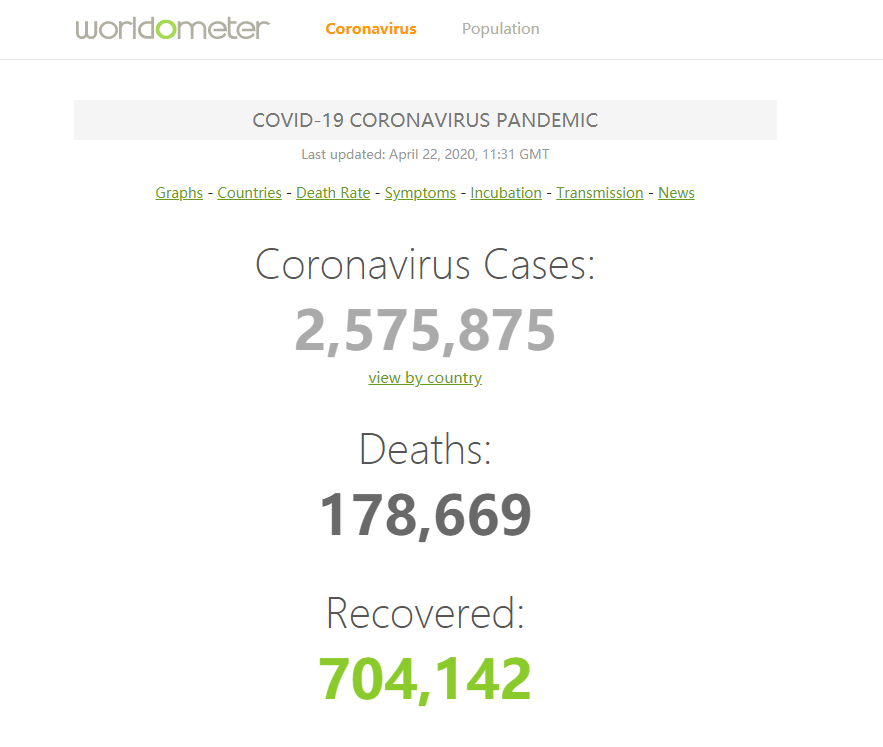
According to the global real-time statistics of Worldmeters, the diagnosed cases of coronavirus disease 2019 (COVID-19) have reached 2, 575, 875.
With the rising of the epidemic data, the global demand for masks is also surging. Every day international logistics companies will receive inquiries about the export freight of masks as follows.
The increase in market demand for masks has attracted more and more enterprises and even individuals to engage in the production of masks. For example, foreign trade companies begin to export masks and foreign businessmen are also busy importing masks.
However, on April 17, the re-upgraded Customs inspection came out. In inspection, the Customs have seized many masks of no name, no address, no production, and sanitation license code.
Masks in the warehouse need to be checked also:
Upon receipt of such information, the purchasers, not ensuring whether masks conform to the standard and are still available, hesitates to import masks from China.
In fact, don’t panic. Sure, all exported masks shall be in good quality to guarantee the conformity of mask quality to export standard and make a contribution to the world.
In the past two months, influenced by the increasing rise of air transport prices and constantly changing Customs policies, the logistics industry and foreign trade manufacturers have been in dilemma. We volunteer to share some problems based on our recent shipment experience.
- Standards of Masks
What many of our customers are worried most is the mask type that can be permitted to export. In fact, masks in all types are permitted. China stays confirmed to encourage the export of anti-epidemic materials rather than restricting and set up checkpoints to strictly observe the quality of exported materials. The manufacturers shall provide corresponding documents for verification. At present, the required documents and material for exported differ according to civil and medical use. The following are perform standard of some masks, only for reference.
| EN-149 | Civil use |
| EN-143 | Civil use |
| EN-14683 | Medical use |
| YY/T 0969-2013 | Disposable medical use |
| YY 0469-2011 | Medical use |
| GB 19083-2010 | Medical use |
| GB 2626-2006 | Civil use |
| GB/T32160 | Civil use |
| With FDA mark (ASTM F2100:19) | Medical use |
| With the description of MEDICAL/SURGICAL on package | Medical use |
You can judge whether the masks are for medical use or for civil use by referring to the performance standards on product qualification certificate and the standards on the inspection report or CE certification. Of course, special cases still exist. Although the inspection report and product qualification certificate issued in China show the masks for civil use, the Customs also check the CE standard regardless of the CE mark on the outer package or inner package. If the CE standard shows masks for medical use, such masks are not permitted to export.

- Necessary Documents
On April 10, the Customs General Administration has issued 2020 No. 53 Notice. In this notice, the Customs decides to implement the legal commodity inspection on 11 varieties of exported commodities including medical masks, which immediately attracts the attention and discussion of the majority of export enterprises and logistics peers. In this article, the present information and the most qualified document format needed in exporting mask are shared with you.

Requirements for non-medical masks
- Offer photographs/ brand/packing list/business license (within business scope)
- Information of manufacturer (name/address/phone number) shall be given on the master carton/product package or product qualification certificate.
- Each small package/box of goods must be attached to the product qualification certificate(stamped with the QC seal and the official seal of the enterprise). The product or package or product qualification certificate shall have: perform standard, production batch, manufacturer’s name, manufacturer’s address, production date, validity period (not one of this element can be dispensed with). The following shows the standard version: (the printed version is required, and the sticky paper version is invalid)
- For non-medical masks, offer product qualification certificates. The template is as follows, which shall be sealed by the manufacturer.
- Domestic qualified inspection report

Requirements for medical masks
- Offer photographs/ brand/packing list/business license (within the business scope and with registration )
- Medical Instrument Production Permit
- Medical Instrument Registration Certificate
- Manufacturer’s qualified inspection report
- The manufacturer and the name and model on the registration certificate shall correspond to the Customs declaration information, and the manufacturing company shall be in the Customs registration list (the registration number shall be valid only when the information of the manufacturing company can be found on the website of National Medical Products Administration)
- Each small package/box of goods must be attached to the product qualification certificate(stamped with the QC seal and the official seal of the enterprise). The product or package or product qualification certificate shall have: perform standard, production batch, manufacturer’s name, manufacturer’s address, production date, validity period (not one of this element can be dispensed with).
Important Notice:
For those illegal or dishonest enterprises that evade Customs inspection by means of the false report, concealment, entrapment or entrainment, or export adulterated, fake, inferior or substandard medical materials, the Customs will not only impose administrative penalties but also transfer them to the judicial organ for criminal responsibility In line with the criminal filing standards.
For legal enterprises that declare truthfully, the Customs will, as always, provide Customs clearance convenience!
You can use this information as a checklist to confirm with your supplier, to get rid of any risk for letting your goods be held by the custom.




This Post Has 0 Comments